
MILLING EQUIPMENT FOR PHARMACEUTICAL MANUFACTURERS
Pharma Milling Equipment for Size Reduction
Advice and best practice articles from our team of experts discussing pharma milling machines.
13 Min Read Time
Selecting the Right Pharmaceutical Milling Equipment
Choosing the right equipment for your pharmaceutical milling process has a significant impact on your process efficiency and the quality, efficacy, and consistency of the final product.
Established in 1976, the Quadro® Comil® has been the industry standard for almost half a century, earning a trusted reputation for quality, innovation, and reliability. Quadro mills are designed to safely handle pharmaceutical powders and heat-sensitive products, which keeps dust generation low.
The Comil® is ideal for a range of processes involved in pharmaceutical manufacturing, including wet granulation, tablet reclaim, dry granulation, and ingredient conditioning.
.webp)
1.1 EFFICIENT CLEANING METHODS
Cleaning Techniques for Particle Sizing and Milling Equipment
In pharmaceutical manufacturing, strict cleaning processes are required to prevent contamination and comply with regulations.
Maintaining rigorous cleaning standards for milling and particle sizing equipment is fundamental to ensuring product quality, meeting regulatory requirements, and protecting your operation from costly contamination risks. In this article we outline proven methods and practical tips to enhance cleaning efficiency while safeguarding compliance.
.webp)
1.2 CHOOSING THE RIGHT MILL SCREEN
Choosing the Correct Mill Screen for Your Bulk Powder Solids
Adjusting parameters like screen type, size, and impeller speed helps achieve the desired particle size and minimize waste.
Key considerations when choosing a conical mill screen include hole type & size, impeller arm profile, impeller tip velocity and RPM, and feeding method. Gain practical insights about selecting the right screen and milling parameters to help you maintain high product quality and optimize your manufacturing workflow.
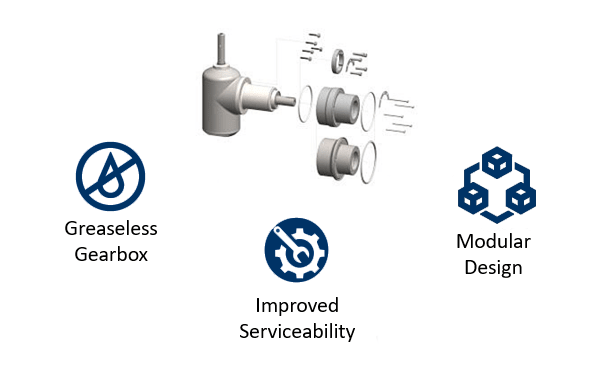
1.3 COMPLIANT PHARMACEUTICAL MANUFACTURING
cGMP Milling with Low Heat and No Contamination
Greaseless gearboxes ensure product integrity, reduce the need for frequent maintenance, and support streamlined cleaning protocols.
Whilst not a common occurrence, some mills can contaminate the final product with grease if the shaft seal becomes worn or damaged, resulting in high product wastage. Greaseless gearboxes support cleaner processing environments and help manufacturers comply with rigorous hygiene standards by eliminating the risk of grease contamination. This leads to greater operational efficiency, lower production costs, and enhanced confidence in the quality and safety of your pharmaceutical products.
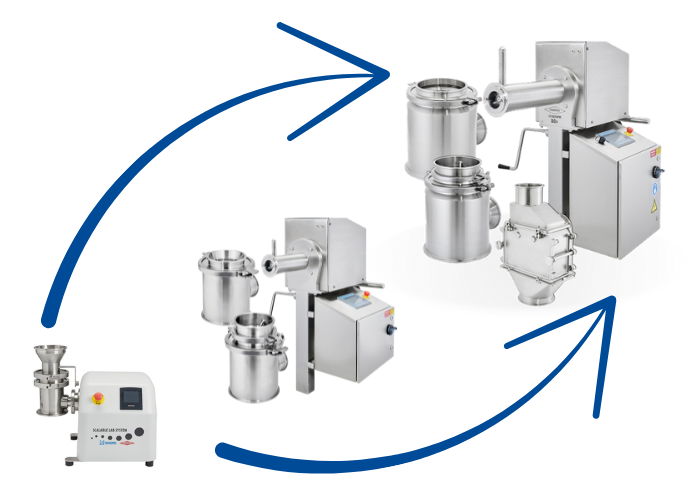
1.4 SCALING UP PRODUCTION
How to Scale Up Your Milling Process as Production Needs Increase
What are the most important factors to evaluate when scaling up to larger volumes?
Pharmaceutical API Milling Equipment
Ensure superior product quality, precise particle size, high output, and operator safety with specialized milling equipment for API production.
The API market is expected to continue growing rapidly as an aging population and greater demand for specialty and biopharmaceutical products drive new opportunities. To stay competitive, API manufacturers must navigate pressure for efficient, high-quality processes while producing APIs with increasingly fine and consistent particle sizes. This requires equipment that ensures operator safety, supports environmental responsibility, and meets stringent cleanliness and product standards.

2.1 IMPORTANCE OF PSD
Why is Particle Size Distribution Important in API Pharmaceuticals?
Controlling particle size is essential to API bioavailability and shelf life; smaller, consistent particles are increasingly more desirable.
In pharmaceutical manufacturing, controlling particle size distribution (PSD) is crucial to the dissolution rate, bioavailability, and shelf life of active pharmaceutical ingredients (APIs). When it is not properly controlled, manufacturers can face issues such as poor flowability, inconsistent drug release, and reduced product stability, which may lead to failed batches, increased waste, and compliance risks. This is especially important for potent compounds and complex formulations where even minor variations can impact efficacy and safety.
In this blog, we outline the key considerations and processes for managing particle size distribution in active pharmaceutical ingredients.

2.2 THE QUADRO FINE GRIND
Effective Fine Particle Size Reduction and Milling
Achieve advanced size reduction with minimum fines and overs as compared to traditional methods.
The Quadro Fine Grind Model F10 is an advanced milling solution that delivers superior fine particle size reduction with fewer fines and overs compared to traditional methods. It enables pharmaceutical manufacturers to achieve greater efficiency, precision, and consistency in producing high-quality powders.
Pharmaceutical OSD Milling Equipment
Achieving the right Particle Size Distribution (PSD) and bulk density for efficient tablet compression.
Producing pharmaceutical oral solid dosage (OSD) products requires careful selection of processes and equipment to overcome the common challenges of meeting patient expectations for quality, bioavailability, taste, and shelf life. To stay competitive, manufacturers need robust, flexible, and cost-effective solutions that deliver high efficiency, reduce downtime and waste, comply with regulatory standards, and ensure consistent product quality and safety.
View our comprehensive milling and size reduction solutions for the wet granulation process via the button below.
.webp)
3.1 WHAT IS WET GRANULATION
Powder Milling Technology for Dry and Wet Processes
When to choose wet granulation for your process and its role in achieving optimal particle size for tablet manufacturing.
Wet granulation is an essential technique in tablet manufacturing, used to bind fine powders into larger, uniform granules by introducing a liquid binder. This process enhances particle size distribution, improves flowability, and delivers superior compressibility—critical factors for achieving consistent, high-quality tablets in the food, pharmaceutical, and chemical industries. Read our blog to explore the key benefits of wet granulation and when to choose it for your process.
.webp)
3.2 IMPROVING EFFICIENCY WITH WET GRANULATION
Reducing Drying Times in the Wet Granulation Process
How a Quadro customer improved their wet granulation process.
Read our bulletin to learn how Quadro equipment improved this customer's wet granulation process, streamlining particle sizing and optimizing efficiency by minimizing drying times and enhancing batch consistency.
.webp)
3.3 PHARMACEUTICAL TABLET RECLAIM
Efficient Tablet Reclaim
Avoiding unnecessary waste and expense in OSD manufacturing with tablet reclaim.
Strict quality control standards and stringent Quality Assurance testing in the pharmaceutical OSD process will often result in tablets being judged unfit for sale - they may be too soft, too hard, broken, off-spec, etc. Efficient tablet reclaim with the Quadro® Comil® in pharmaceutical manufacturing can help reduce material loss and overall production costs.

3.4 DRY GRANULATION APPLICATION BULLETIN
Dry Granulation Sizing Prior to Tableting
Increasing production capacity when producing pharma OSD products.
By integrating an in-line Quadro Comil, this customer more than doubled their production capacity, efficiently reducing oversized particles from the blender while maintaining consistent particle size distribution in the rest of the material.
LEARN MORE
Pharmaceutical Milling Solutions
Get in touch with the Quadro team to benefit from our expertise in pharmaceutical process solutions. Whether you need support with selecting the correct mill, screen, speed or capacity, our application team is on-hand to provide best practices and advice.



